Vacuum Technology
Vacuum technology
What is a vacuum pump?
To create a vacuum in a system it is necessary to move all of the molecules of gas out of the system. The molecules will move only if there is a pressure difference between the two regions of the space (see Figure 4). The low pressure region is the space with the smaller number of molecules, while the high pressure region is the space with the larger number of molecules.
Any device which can induce a pressure difference between the two regions in the space is called a pump. The pump which creates the vacuum in the certain system is called a vacuum pump.
Exercise: you need only
a cup of beverage and a straw. Try to suck a drink by the straw and
feel how do the mouth muscles move. When one sucks on a straw the mouth
muscles create the region of low pressure, while atmospheric pressure
on the surface of your beverage pushes it up the straw. Pumps operate
on the same principles.

Figure 4: Vacuum pump operation.
How
does the vacuum pump operate?
There are two categories of vacuum pumps: transfer pumps and trapping pumps.
The transfer pumps are
also called kinetic pumps since they impart the momentum to the
gas which is being pushed in a such a way that the gas is transferred
continuously from the inlet of the pump to the outlet. This is usually
done by mechanical moving parts of the pump, as shown in Figure 5. The
moving (usually rotating) part of the pump accelerates the molecules of
the gas and makes the region of lower pressure. Therefore, the molecules
from the tank will start moving towards the region of lower static pressure,
with the procedure continuously repeating until all (or most) of the molecules
are taken from the container where we would like to have a vacuum. When
we get the wanted level of vacuum we isolate the tank by a high vacuum
valve. This valve stops any exchange of gas between the container and
the pump.
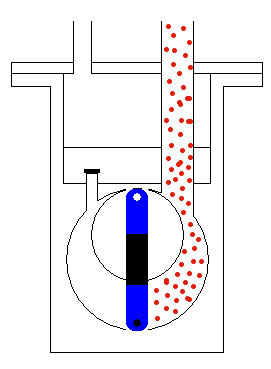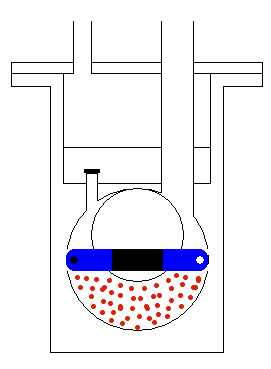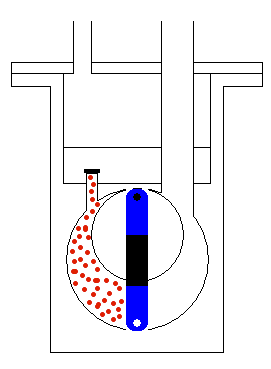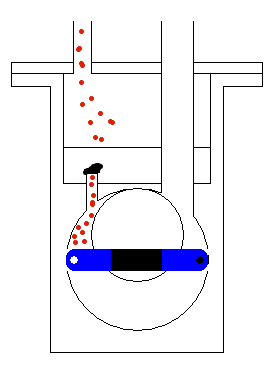
Figure 5: Operation of the transfer pump.
The trapping or capture
pumps are usually located in the container being evacuated. The trapping
pumps remove gas molecules by sorption or condensation on its internal
surfaces (see Figure 6). If the gas molecules chemically react with the
internal material of the pump, the new material created by the reaction
of gas and inner material molecules will be deposited as a thin film.
This is called sorption of the gas molecules. Furthermore, if the gas
molecules come in touch with the refrigerated surfaces of the pump, gas
will be condensed and removed as a liquid.
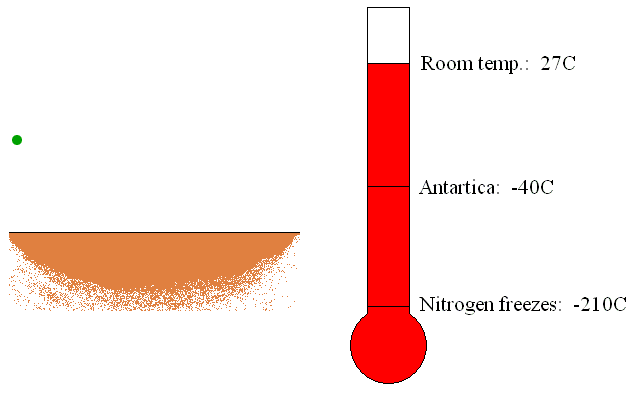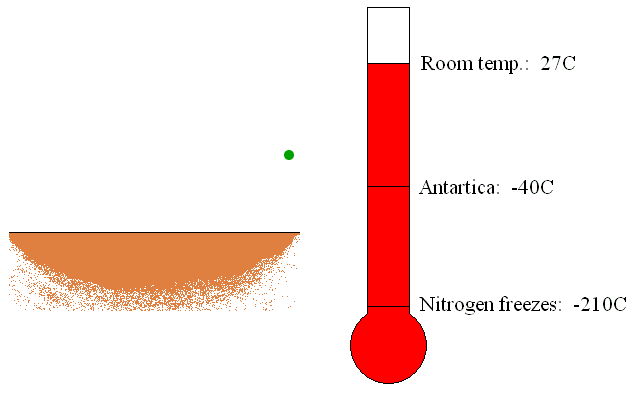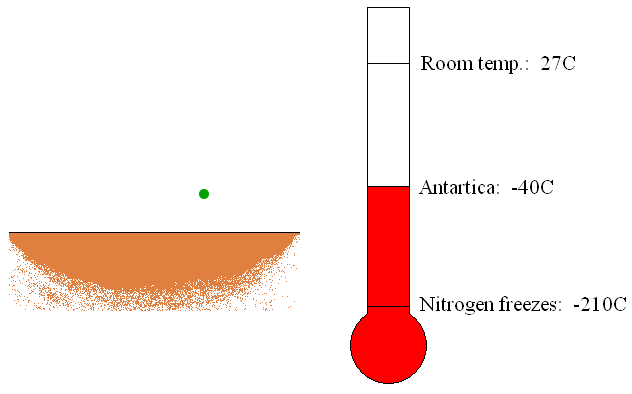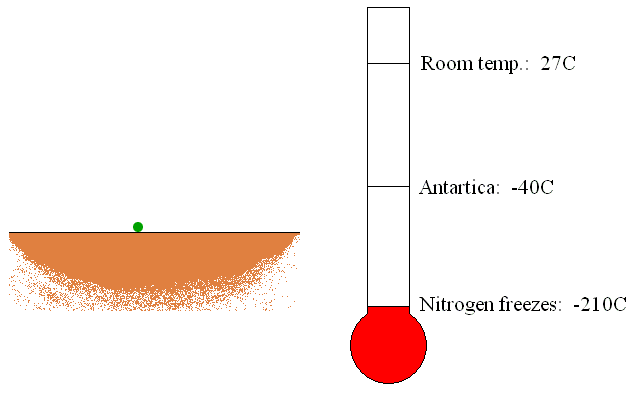
Figure 6: Operation of the trapping vacuum pump.
How do we measure the vacuum?
The low vacuum levels can be
measured also with the simplest manometer; i.e.; U � tube (see again Figure
2). In this case, instead of having a container with pressurized gas,
we will have a vacuum container. Since the atmospheric pressure is higher,
the air molecules on the open end of U � tube will push the fluid towards
vacuum container. Again, the difference in heights of fluid between the
two sides of U � tube will determine how much the pressure in the container
is less than the atmospheric pressure.
In general, the special devices which measure the vacuum levels are called vacuum gauges.
The simplest mechanical gauge
you can imagine as a chamber divided by a membrane. One side of the chamber
is connected to the vacuum container and the other to the known pressure
tank (it can be simply open, therefore it will be under the atmoshperic
pressure). The membrane is attached to the pointer mechanism (see Figure
7). Depending on the membrane deformation, the pointer will show the vacuum
level. A gauge like this is considered to be a coarse or rough guage.
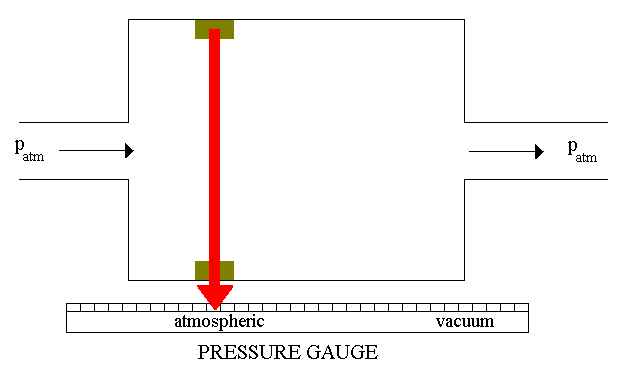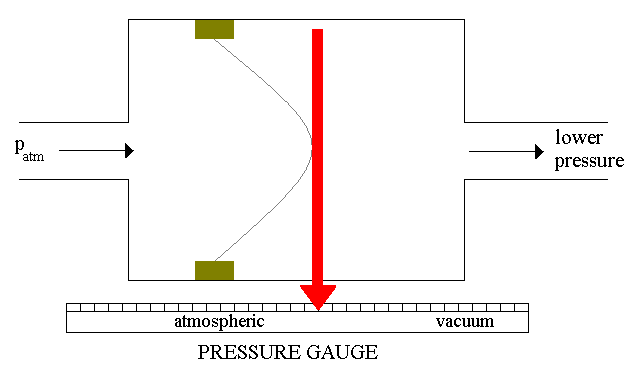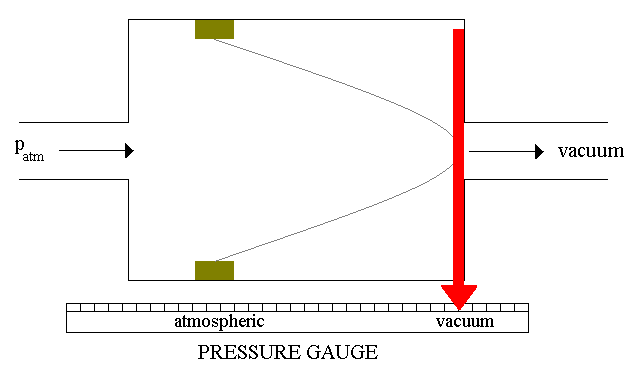
Figure 7: Vacuum gauge.
Continue on to the experiments with vacuum
Page authored by Rajka Krstic, Jennifer Trelewicz and Veena Mahesh
Copyright © 1995-2000 Arizona Board of Regents. All rights reserved.