
O-Chem in Real Life: Soaps and Detergents
The definition of a carboxylic acid derivative is a compound that hydrolyzes (usually with an acid or base catalyst) to a carboxylic acid. An ester hydrolyzes to a carboxylic acid and an alcohol. If this hydrolysis is performed in the presence of base, then the anionic form of the carboxylic acid is formed, the carboxylate anion.

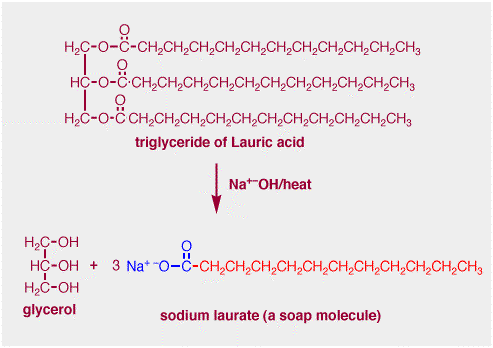
Fat molecules are a form of ester. Ester formation is the reverse of ester hydrolysis. The trihydroxy compound glycerol shown below forms triesters with the so-called fatty carboxylic acids, i.e. the acids structures that are found in fats. Fatty acids have long hydrocarbon chains containing anywhere from 4 to 18 carbons. The example below shows a fat molecule that consists of three molecules of Lauric acid (which has a 12 carbon chain) combined with glycerol. Molecules of this kind are called triglycerides. (If a triglyceride is a liquid at room temperature it is an oil.) There are a very large number of fatty acids, some are saturated (the hydrocarbon chain contains no double bonds) and some are unsaturated (the chain does contain double bonds).
So, hydrolysis of a fat under basic conditions, such as with hydroxide, generates glycerol and three molecules of the fatty acid carboxylate. It is these carboxlyates that are the soap molecules, and historically this is exactly how soap was made, i.e. by "cooking" fats with sodium hydroxide in the form of lye.
A soap molecule consists of a polar ionic hydrophilic (water "loving") end, which is shown in blue in the structure above, and a non-polar hydrophobic (water "hating") end, which is the hydrocarbon chain shown in red above. When dissolved in water the soap molecules arrange themselves in the form of roughly spherical aggregates of 60 or so molecules, called micelles. Micelles have the hydrophobic tails clustered together in the center (away from the water), and the hydrophilic ionic ends are on the outside, and are solvated by the water molecules. Micelles thus represent tiny hydrophobic pockets floating around in water, and can solubilize other hydrophobic molecules, such as oils etc. This is the usual description of how soap aids in cleaning, i.e. by dissolving substances that otherwise do not dissolve in water, although I am sure that it is more complex than this!

Soap molecules have problems in hard water. Hard water contains minerals in the form of salts, for example that from the SALT RIVER!! Magnesium and calcium salts are particularly problematic. Although sodium carboxylates are water soluble, calcium and magnesium carboxylates tend to be much less water soluble. Adding soap to water hard water forms soap "scum", i.e. precipitates of calcium and magnesium carboxylates.
One solution to the soap scum problem is to alter the nature of the soap molecule. This is where detergents come in. Detergents were actually developed in response to a shortage of animal and vegetable fats during WW I and WW II, the soap scum thing was an added benefit. Detergents are artifical soap molecules that are industrially synthesized, rather than obtained from natural sources. They have the same general structure, i.e. a hydrophobic end and a hydrophilic end. In most detergents the hydrophilic end is ionic, as in soaps, but in others it is a polar organic fragment. A typical structure is shown below with a sulfonate as the ionic part.

Calcium and magnesium salts of sulfonates are water soluble, thus the soap scum problem is solved! There is a problem, however. Since soaps are natural products they are fairly biodegradable, whereas the non-natural detergents are often not biodegradable, leading to foamy looking polluted water. Chemists are currently working on more efficient detergents that do biodegrade.
I once did quite a lot of work on photochemical reactions in soaps and related structures. Because many organic molecules locate themselves inside hydrophobic interiors of micelles, they can act as tiny reactors or cages for organic molecules. These tiny reactors could be used to alter the course of and control many chemical reactions in interesting ways.